Prokaryotic cells are about 10 times smaller than eukaryotic cells. A typical E. coli cell is about 1 μm wide and 2 to 3μm long. Structurally, prokaryotes are very simple cells when compared with eukaryotic cells, and yet they are able to perform the necessary processes of life. Reproduction of prokaryotic cells is by binary fission—the simple division of one cell into two cells, after DNA replication and the formation of a separating membrane and cell wall. All bacteria are prokaryotes, as are the archaea.
Embedded within the cytoplasm of prokaryotic cells are a chromosome, ribosomes, and other cytoplasmic particles (Fig. 1). Unlike eukaryotic cells, the cytoplasm of prokaryotic cells is not filled with internal membranes. The cytoplasm is surrounded by a cell membrane, a cell wall (usually), and sometimes a capsule or slime layer. These latter three structures make up the bacterial cell envelope. Depending on the particular species of bacterium, flagella, pili (description follows), or both may be observed outside the cell envelope, and a spore may sometimes be seen within the cell.
Many enzymes are attached to the cell membrane, and various metabolic reactions take place there. Some scientists believe that inward foldings of the cell membranes—called mesosomes—are where cellular respiration takes place in bacteria. This process is similar to that which occurs in the mitochondria of eukaryotic cells, in which nutrients are broken down to produce energy in the form of ATP molecules. On the other hand, some scientists think that mesosomes are nothing more than artifacts created during the processing of bacterial cells for electron microscopy.
In cyanobacteria and other photosynthetic bacteria (bacteria that convert light energy into chemical energy), infoldings of the cell membrane contain chlorophyll and other pigments that serve to trap light energy for photosynthesis. However, prokaryotic cells do not have complex internal membrane systems similar to the ER and Golgi complex of eukaryotic cells. Prokaryotic cells do not contain any membrane-bound organelles or vesicles.
The thin and tightly folded chromosome of E. coli is about 1.5 mm (1,500 μm) long and only 2 nm wide. Because a typical E. coli cell is about 2 to 3 μm long, its chromosome is approximately 500 to 750 times longer than the cell itself—quite a packaging feat! Bacterial chromosomes contain between 575 and 55,000 genes, depending on the species. Each gene codes for one or more gene products (enzymes, other proteins, and rRNA and tRNA molecules). In comparison, the chromosomes within a human cell contain between 20,000 and 25,000 genes.
Small, circular molecules of double-stranded DNA that are not part of the chromosome (referred to as extra-chromosomal DNA or plasmids) may also be present in the cytoplasm of prokaryotic cells (Fig. 2). A plasmid may contain anywhere from fewer than 10 genes to several hundred genes. A bacterial cell may not contain any plasmids, or it may contain one plasmid, multiple copies of the same plasmid, or more than one type of plasmid (i.e., plasmids containing different genes). Plasmids have also been found in yeast cells.
Beware of similar sounding words - A plasmid is a small, circular molecule of double-stranded DNA. It is referred to as extrachromosomal DNA because it is not part of the chromosome. Plasmids are found in most bacteria. A plastid is a cytoplasmic organelle, found only in certain eukaryotic cells (e.g., algae and plants). Plastids are the sites of photosynthesis.
Cytoplasmic Particles Within the bacterial cytoplasm, many tiny particles have been observed. Most of these are ribosomes, often occurring in clusters called polyribosomes or polysomes (poly meaning many). Prokaryotic ribosomes are smaller than eukaryotic ribosomes, but their function is the same—they are the sites of protein synthesis. A 70S prokaryotic ribosome is composed of a 30S subunit and a 50S subunit. It has been estimated that there are about 15,000 ribosomes in the cytoplasm of an E. coli cell.
Cytoplasmic granules occur in certain species of bacteria. These may be stained by using a suitable stain, and then identified microscopically. The granules may consist of starch, lipids, sulfur, iron, or other stored substances.
Some bacteria lose their ability to produce cell walls, transforming into tiny variants of the same species, referred to as L-form or cell wall–deficient (CWD) bacteria. Over 50 different species of bacteria are capable of transforming into CWD bacteria, some of which might be responsible for chronic diseases such as chronic fatigue syndrome, Lyme disease, rheumatoid arthritis, and sarcoidosis. Clinicians are often unaware that CWD bacteria are present in their patients because they will not grow under standard laboratory conditions; they must be cultured in a different medium and at a different temperature than typical bacteria.
The other type of glycocalyx, called a capsule, is highly organized and firmly attached to the cell wall. Capsules usually consist of polysaccharides, which may be combined with lipids and proteins, depending on the bacterial species. Knowledge of the chemical composition of capsules is useful in differentiating among different types of bacteria within a particular species; for example, different strains of the bacterium H. influenzae, a cause of meningitis and ear infections in children, are identified by their capsular types. A vaccine, called Hib vaccine, is available for protection against disease caused by H.influenzae capsular type b. Other examples of encapsulated bacteria are Klebsiella pneumoniae, Neisseria meningitidis, and Streptococcus pneumoniae.
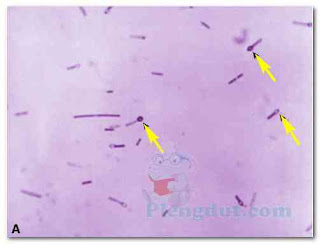
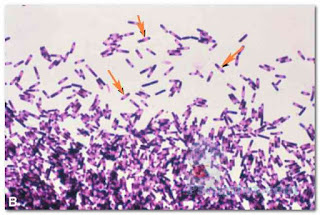
Capsules can be detected using a capsule staining procedure, which is a type of negative stain. The bacterial cell and background become stained, but the capsule remains unstained (Fig. 5). Thus, the capsule appears as an unstained halo around the bacterial cell. Antigen–antibody tests may be used to identify specific strains of bacteria possessing unique capsular molecules (antigens).
Encapsulated bacteria usually produce colonies on nutrient agar that are smooth, mucoid, and glistening; they are referred to as S-colonies. Nonencapsulated bacteria tend to grow as dry, rough colonies, called R-colonies. Capsules serve an antiphagocytic function, protecting the encapsulated bacteria from being phagocytized (ingested) by phagocytic white blood cells. Thus, encapsulated bacteria are able to survive longer in the human body than nonencapsulated bacteria.
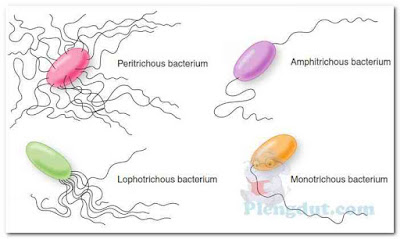
The number and arrangement of flagella possessed by a certain species of bacterium are characteristic of that species and can, thus, be used for classification and identification purposes (Fig. 6). Bacteria possessing flagella over their entire surface (perimeter) are called peritrichous bacteria. Bacteria with a tuft of flagella at one end are described as being lophotrichous bacteria, whereas those having one or more flagella at each end are said to be amphitrichous bacteria. Bacteria possessing a single polar flagellum are described as monotrichous bacteria. In the laboratory, the number of flagella that a cell possesses and their locations on the cell can be determined using what is known as a flagella stain. The stain adheres to the flagella, making them thick enough to be seen under the microscope (Fig. 7).
Bacterial flagella consist of three, four, or more threads of protein (called flagellin) twisted like a rope. Thus, the structures of bacterial flagella and eukaryotic flagella are quite different. You will recall that eukaryotic flagella (and cilia) contain a complex arrangement of internal microtubules, which run the length of the membranebound flagellum. Bacterial flagella do not contain microtubules, and their flagella are not membrane-bound. Bacterial flagella arise from a basal body in the cell membrane and project outward through the cell wall and capsule (if present), as was shown in Figure 1.
Some spirochetes (spiral-shaped bacteria) have two flagella-like fibrils called axial filaments, one attached to each end of the bacterium. These axial filaments extend toward each other, wrap around the organism between the layers of the cell wall, and overlap in the midsection of the cell. As a result of its axial filaments, spirochetes can move in a spiral, helical, or inchworm manner.
The pili that merely enable bacteria to anchor themselves to surfaces (e.g., tissues within the human body) are usually quite numerous (Fig. 8). In some species of bacteria, piliated strains (those possessing pili) are able to cause diseases such as urethritis and cystitis, whereas nonpiliated strains (those not possessing pili) of the same organisms are unable to cause these diseases.
A bacterial cell possessing a sex pilus (called a donor cell)—and the cell only possesses one sex pilus—is able to attach to another bacterial cell (called a recipient cell) by means of the sex pilus. Genetic material (usually in the form of a plasmid) is then transferred from the donor cell to the recipient cell—a process known as conjugation.
Embedded within the cytoplasm of prokaryotic cells are a chromosome, ribosomes, and other cytoplasmic particles (Fig. 1). Unlike eukaryotic cells, the cytoplasm of prokaryotic cells is not filled with internal membranes. The cytoplasm is surrounded by a cell membrane, a cell wall (usually), and sometimes a capsule or slime layer. These latter three structures make up the bacterial cell envelope. Depending on the particular species of bacterium, flagella, pili (description follows), or both may be observed outside the cell envelope, and a spore may sometimes be seen within the cell.
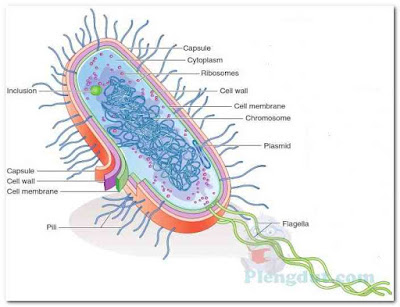 |
| Figure.1: A typical prokaryotic cell. |
Cell Membrane
Capsule Ribosomes Cytoplasm Enclosing the cytoplasm of a prokaryotic cell is the cell membrane (also known as the plasma, cytoplasmic, or cellular membrane). This membrane is similar in structure and function to the eukaryotic cell membrane. Chemically, the cell membrane consists of proteins and phospholipids. Being selectively permeable, the membrane controls which substances may enter or leave the cell. It is flexible and so thin that it cannot be seen with a compound light microscope. However, it is frequently observed in transmission electron micrographs of bacteria.Many enzymes are attached to the cell membrane, and various metabolic reactions take place there. Some scientists believe that inward foldings of the cell membranes—called mesosomes—are where cellular respiration takes place in bacteria. This process is similar to that which occurs in the mitochondria of eukaryotic cells, in which nutrients are broken down to produce energy in the form of ATP molecules. On the other hand, some scientists think that mesosomes are nothing more than artifacts created during the processing of bacterial cells for electron microscopy.
In cyanobacteria and other photosynthetic bacteria (bacteria that convert light energy into chemical energy), infoldings of the cell membrane contain chlorophyll and other pigments that serve to trap light energy for photosynthesis. However, prokaryotic cells do not have complex internal membrane systems similar to the ER and Golgi complex of eukaryotic cells. Prokaryotic cells do not contain any membrane-bound organelles or vesicles.
Chromosome
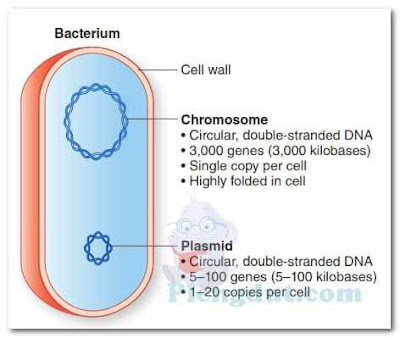
The prokaryotic chromosome usually consists of a single, long, supercoiled, circular DNA molecule, which serves as the control center of the bacterial cell. It is capable of duplicating itself, guiding cell division, and directing cellular activities. A prokaryotic cell contains neither nucleoplasm nor a nuclear membrane. The chromosome is suspended or embedded in the cytoplasm. The DNA-occupied space within a bacterial cell is sometimes referred to as the bacterial nucleoid.The thin and tightly folded chromosome of E. coli is about 1.5 mm (1,500 μm) long and only 2 nm wide. Because a typical E. coli cell is about 2 to 3 μm long, its chromosome is approximately 500 to 750 times longer than the cell itself—quite a packaging feat! Bacterial chromosomes contain between 575 and 55,000 genes, depending on the species. Each gene codes for one or more gene products (enzymes, other proteins, and rRNA and tRNA molecules). In comparison, the chromosomes within a human cell contain between 20,000 and 25,000 genes.
Small, circular molecules of double-stranded DNA that are not part of the chromosome (referred to as extra-chromosomal DNA or plasmids) may also be present in the cytoplasm of prokaryotic cells (Fig. 2). A plasmid may contain anywhere from fewer than 10 genes to several hundred genes. A bacterial cell may not contain any plasmids, or it may contain one plasmid, multiple copies of the same plasmid, or more than one type of plasmid (i.e., plasmids containing different genes). Plasmids have also been found in yeast cells.
Cytoplasm
The semiliquid cytoplasm of prokaryotic cells consists of water, enzymes, dissolved oxygen (in some bacteria), waste products, essential nutrients, proteins, carbohydrates, and lipids a complex mixture of all the materials required by the cell for its metabolic functions. There is some evidence to suggest that bacterial cytoplasm contains a cytoskeletal structure similar to that of eukaryotic cells.Beware of similar sounding words - A plasmid is a small, circular molecule of double-stranded DNA. It is referred to as extrachromosomal DNA because it is not part of the chromosome. Plasmids are found in most bacteria. A plastid is a cytoplasmic organelle, found only in certain eukaryotic cells (e.g., algae and plants). Plastids are the sites of photosynthesis.
Cytoplasmic Particles Within the bacterial cytoplasm, many tiny particles have been observed. Most of these are ribosomes, often occurring in clusters called polyribosomes or polysomes (poly meaning many). Prokaryotic ribosomes are smaller than eukaryotic ribosomes, but their function is the same—they are the sites of protein synthesis. A 70S prokaryotic ribosome is composed of a 30S subunit and a 50S subunit. It has been estimated that there are about 15,000 ribosomes in the cytoplasm of an E. coli cell.
Cytoplasmic granules occur in certain species of bacteria. These may be stained by using a suitable stain, and then identified microscopically. The granules may consist of starch, lipids, sulfur, iron, or other stored substances.
Bacterial Cell Wall
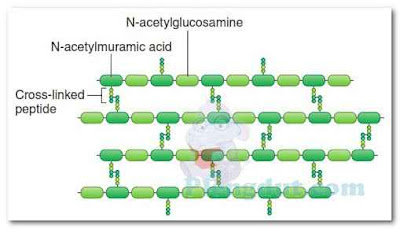
The rigid exterior cell wall that defines the shape of bacterial cells is chemically complex. Thus, the structure of bacterial cell walls is quite different from the relatively simple structure of eukaryotic cell walls, although they serve the same functions—providing rigidity, strength, and protection. The main constituent of most bacterial cell walls is a complex macromolecular polymer known as peptidoglycan (also known as murein), consisting of many polysaccharide chains linked together by small peptide (protein) chains (Fig. 3). Peptidoglycan is only found in bacteria. The thickness of the cell wall and its exact composition vary with the species of bacteria. The cell walls of certain bacteria, called Gram-positive bacteria, have a thick layer of peptidoglycan combined with teichoic acid and lipoteichoic acid molecules (Fig. 4). The cell walls of Gram-negative bacteria (also explained in Chapter 4) have a much thinner layer of peptidoglycan, but this layer is covered with a complex layer of lipid macromolecules, usually referred to as the outer membrane, as shown in Figure 4. Although most bacteria have cell walls, bacteria in the genus Mycoplasma do not. Archaea (described in Chapter 4) have cell walls, but their cell walls do not contain peptidoglycan.Some bacteria lose their ability to produce cell walls, transforming into tiny variants of the same species, referred to as L-form or cell wall–deficient (CWD) bacteria. Over 50 different species of bacteria are capable of transforming into CWD bacteria, some of which might be responsible for chronic diseases such as chronic fatigue syndrome, Lyme disease, rheumatoid arthritis, and sarcoidosis. Clinicians are often unaware that CWD bacteria are present in their patients because they will not grow under standard laboratory conditions; they must be cultured in a different medium and at a different temperature than typical bacteria.
Glycocalyx (Slime Layers and Capsules)
Some bacteria have a thick layer of material (known as glycocalyx) located outside their cell wall. Glycocalyx is a slimy, gelatinous material produced by the cell membrane and secreted outside of the cell wall. There are two types of glycocalyx. One type, called a slime layer, is not highly organized and is not firmly attached to the cell wall. It easily detaches from the cell wall and drifts away. Bacteria in the genus Pseudomonas produce a slime layer, which sometimes plays a role in diseases caused by Pseudomonas species. Slime layers enable certain bacteria to glide or slide along solid surfaces, and seem to protect bacteria from antibiotics and desiccation.The other type of glycocalyx, called a capsule, is highly organized and firmly attached to the cell wall. Capsules usually consist of polysaccharides, which may be combined with lipids and proteins, depending on the bacterial species. Knowledge of the chemical composition of capsules is useful in differentiating among different types of bacteria within a particular species; for example, different strains of the bacterium H. influenzae, a cause of meningitis and ear infections in children, are identified by their capsular types. A vaccine, called Hib vaccine, is available for protection against disease caused by H.influenzae capsular type b. Other examples of encapsulated bacteria are Klebsiella pneumoniae, Neisseria meningitidis, and Streptococcus pneumoniae.
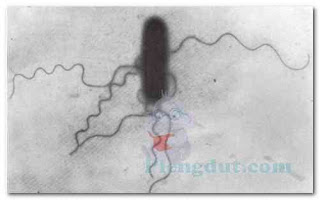
Capsules can be detected using a capsule staining procedure, which is a type of negative stain. The bacterial cell and background become stained, but the capsule remains unstained (Fig. 5). Thus, the capsule appears as an unstained halo around the bacterial cell. Antigen–antibody tests may be used to identify specific strains of bacteria possessing unique capsular molecules (antigens).
Encapsulated bacteria usually produce colonies on nutrient agar that are smooth, mucoid, and glistening; they are referred to as S-colonies. Nonencapsulated bacteria tend to grow as dry, rough colonies, called R-colonies. Capsules serve an antiphagocytic function, protecting the encapsulated bacteria from being phagocytized (ingested) by phagocytic white blood cells. Thus, encapsulated bacteria are able to survive longer in the human body than nonencapsulated bacteria.
Flagella
Flagella (sing., flagellum) are thread-like, protein appendages that enable bacteria to move. Flagellated bacteria are said to be motile, whereas nonflagellated bacteria are usually nonmotile. Bacterial flagella are about 10 to 20 nm thick; too thin to be seen with the compound light microscope.The number and arrangement of flagella possessed by a certain species of bacterium are characteristic of that species and can, thus, be used for classification and identification purposes (Fig. 6). Bacteria possessing flagella over their entire surface (perimeter) are called peritrichous bacteria. Bacteria with a tuft of flagella at one end are described as being lophotrichous bacteria, whereas those having one or more flagella at each end are said to be amphitrichous bacteria. Bacteria possessing a single polar flagellum are described as monotrichous bacteria. In the laboratory, the number of flagella that a cell possesses and their locations on the cell can be determined using what is known as a flagella stain. The stain adheres to the flagella, making them thick enough to be seen under the microscope (Fig. 7).
 |
| Figure.7: Salmonella cells, showing peritrichous flagella. Salmonella is a bacterial genus. The cells were stained using a flagella stain. (Courtesy of the CDC.) |
Bacterial flagella consist of three, four, or more threads of protein (called flagellin) twisted like a rope. Thus, the structures of bacterial flagella and eukaryotic flagella are quite different. You will recall that eukaryotic flagella (and cilia) contain a complex arrangement of internal microtubules, which run the length of the membranebound flagellum. Bacterial flagella do not contain microtubules, and their flagella are not membrane-bound. Bacterial flagella arise from a basal body in the cell membrane and project outward through the cell wall and capsule (if present), as was shown in Figure 1.
Some spirochetes (spiral-shaped bacteria) have two flagella-like fibrils called axial filaments, one attached to each end of the bacterium. These axial filaments extend toward each other, wrap around the organism between the layers of the cell wall, and overlap in the midsection of the cell. As a result of its axial filaments, spirochetes can move in a spiral, helical, or inchworm manner.
Pili (Fimbriae)
Pili (sing., pilus) or fimbriae (sing., fimbria) are hair-like structures, most often observed on Gram-negative bacteria. They are composed of polymerized protein molecules called pilin. Pili are much thinner than flagella, have a rigid structure, and are not associated with motility. These tiny appendages arise from the cytoplasm and extend through the plasma membrane, cell wall, and capsule (if present). There are two types of pili: one type merely enables bacteria to adhere or attach to surfaces; the other type (called a sex pilus) facilitates transfer of genetic material from one bacterial cell to another following attachment of the cells to each other.The pili that merely enable bacteria to anchor themselves to surfaces (e.g., tissues within the human body) are usually quite numerous (Fig. 8). In some species of bacteria, piliated strains (those possessing pili) are able to cause diseases such as urethritis and cystitis, whereas nonpiliated strains (those not possessing pili) of the same organisms are unable to cause these diseases.
A bacterial cell possessing a sex pilus (called a donor cell)—and the cell only possesses one sex pilus—is able to attach to another bacterial cell (called a recipient cell) by means of the sex pilus. Genetic material (usually in the form of a plasmid) is then transferred from the donor cell to the recipient cell—a process known as conjugation.


![Capsule staining. A. Drawing illustrating the results of the capsule staining technique. B. Photomicrograph of encapsulated bacteria that have been stained using the capsule staining technique. The capsule staining is an example of a negative staining technique. Note that the bacterial cells and the background stain, but the capsules do not. The capsules are seen as unstained “halos” around the bacterial cells. ([B] From Winn WC Jr, et al. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.) Capsule staining. A. Drawing illustrating the results of the capsule staining technique. B. Photomicrograph of encapsulated bacteria that have been stained using the capsule staining technique. The capsule staining is an example of a negative staining technique. Note that the bacterial cells and the background stain, but the capsules do not. The capsules are seen as unstained “halos” around the bacterial cells. ([B] From Winn WC Jr, et al. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.)](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgpgHX6yZ21NED7p2MJy66vJvVCxVDUN6u3jmTrUeZnR7DV4Y-C71-bCYVlXs9EPsTJlex-QuLj48gc5h9RCYJW6iLSPLKiPuBk-noe5kkdXO9lmVcnb-PYGCPhb1rw4nxjqmPasny271j2/s400/2016-06-07_082412.jpg)